For citation analyses and h-index check Google Scholar.
2024
77. A large-scale machine learning analysis of inorganic nanoparticles in preclinical cancer research
B. B. Mendes, Z. Zhang, J Conniot, D. P Sousa, J. M. J. M. Ravasco, L. A. Onweller, A. Lorenc, T Rodrigues*, D Reker*, J Conde*
Nature Nanotechnol 2024, accepted
76. Machine learning uncovers natural product modulators of the 5-lipoxygenase pathway and facilitates elucidation of their biological mechanisms
S. Mikutis, S. Lawrinowitz, C. Kretzer, L. Dunsmore, L. Skeretis, T. Rodrigues, O. Werz, G. Bernardes
ACS Chem Biol 2024, 19, 217220
2023
75. Chemistry automated by large language models
A. L. Dias, T. Rodrigues*
Nature 2023, 624, 530–531
74. The rise of automated curiosity-driven discoveries in chemistry
L. Bustillo, T. Laino, T. Rodrigues*
Chem Sci 2023, 14, 10378-10384
73. Merging the Isonitrile–Tetrazine (4+1) Cycloaddition and the Ugi Four-Component Reaction into a Single Multicomponent Process
Y. Méndez, A. V. Vasco, G. Ivey, P. Gierth, B. B. Sousa, C. D. Navo, A. L. Dias, A. Torres-Mozas, T. Rodrigues, G. Jiménez-Osés, G. J. L. Bernardes
Angew Chem Int Ed 2023, 62, e2023111
72. Limitations of representation learning in small molecule property prediction
A. L. Dias, L. Bustillo, T. Rodrigues*
Nat Commun 2023, 14, 6394
71. A focus on the use of real-world datasets for yield prediction
L. Bustillo, T. Rodrigues*
Chem Sci 2023, 14, 4958-4960
2022
70. Chemoproteomics-Enabled Identification of 4-Oxo-β-Lactams as Inhibitors of Dipeptidyl Peptidases 8 and 9
L. A. R. Carvalho, B. Ross, L. Fehr, O. Bolgi, S. Wöhrle, K. M. Lum, D. Podlesainski, A. C. Vieira, R. Kiefersauer, R. Félix, T. Rodrigues, S. D. Lucas, O. Groß, R. Geiss-Friedlander, B. F. Cravatt, R. Huber, M. Kaiser, R. Moreira
Angew Chem Int Ed 2022, 61, e202213804
69. A Special issue on artificial intelligence for drug discovery
T. Rodrigues*
Bioorg Med Chem 2022, 70, 116939
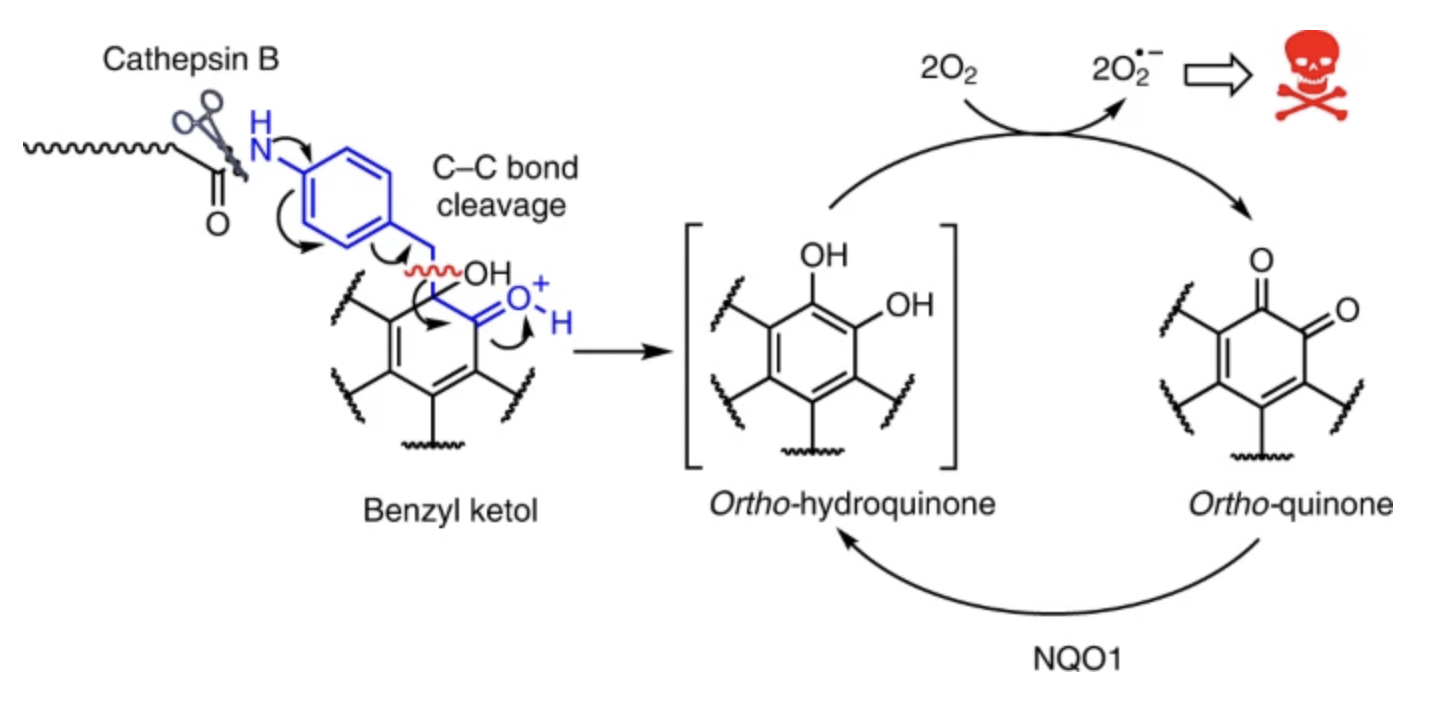
68. Controlled masking and targeted release of redox-cycling ortho-quinones via a C–C bond-cleaving 1,6-elimination
L. Dunsmore, C. D. Navo, J. Becher, E. G. de Montes, A. Guerreiro, E. Hoyt , L. Brown, V. Zelenay, S. Mikutis, J. Cooper, I. Barbieri, S. Lawrinowitz, E. Siouve, E. Martin, P. R. Ruivo, T. Rodrigues, F. P. da Cruz, O. Werz, G. Vassiliou, P. Ravn, G. Jiménez-Osés, G. J. L. Bernardes
Nat Chem 2022, 14, 754-765
67. Evaluation guidelines for machine learning tools in the chemical sciences
A. Bender, N. Schneider, M. Segler, W. P. Walters, O. Engkvist, T. Rodrigues*
Nat Rev Chem 2022, 6, 428-442

66. Nuisance small molecules under a machine-learning lens
T. Rodrigues*
Digital Discov 2022, 1, 209-2015
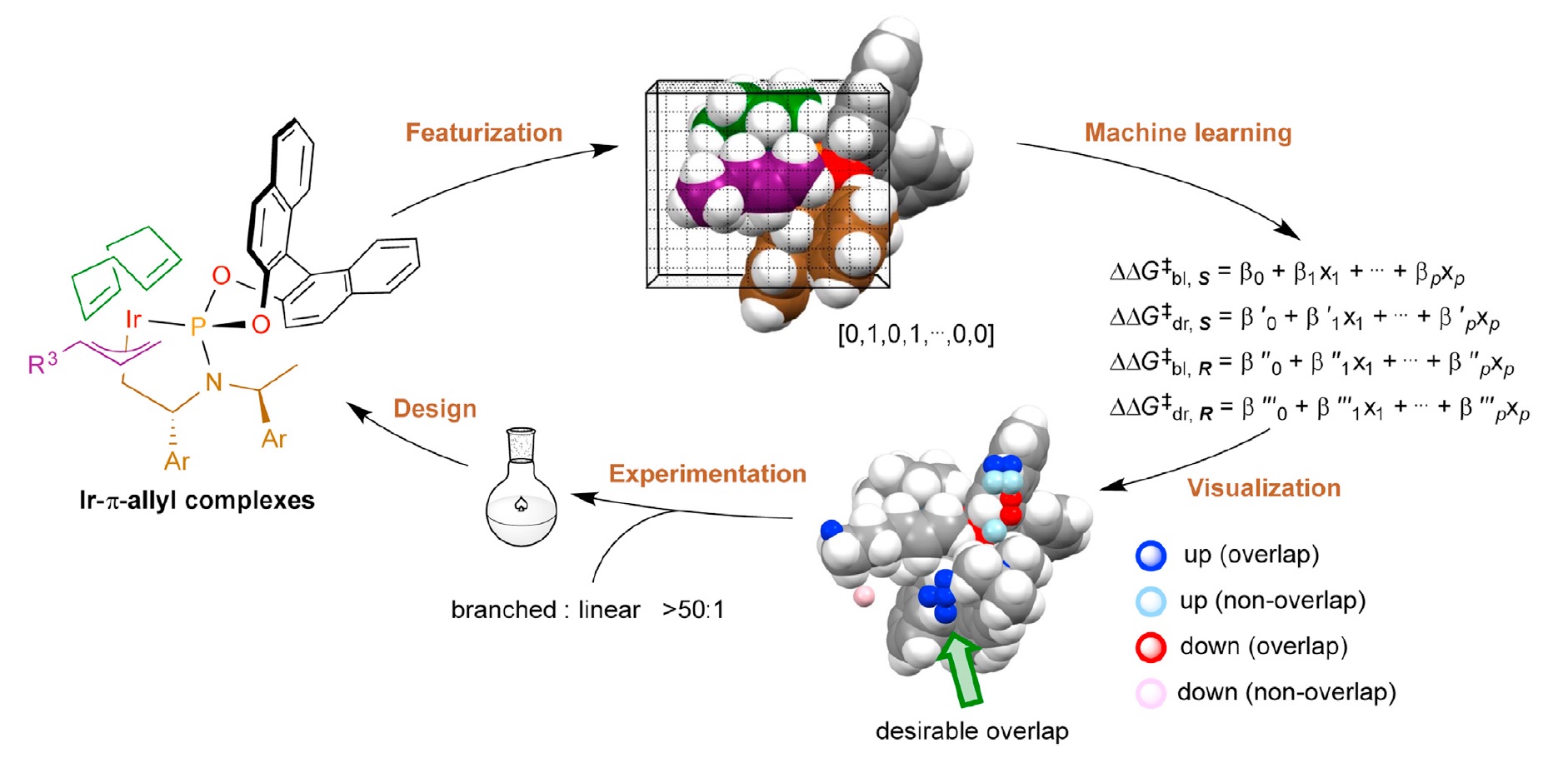
65. Deriving intuition in catalyst design with machine learning
T. Rodrigues*
Chem 2022, 8, 15-19
2021
64. Machine learning for next generation nanotechlonology in healthcare
A. Lorenc, B. B. Mendes, J. Conniot, D. P. Sousa, J. Conde*, T. Rodrigues*
Matter 2021, 4, 3078-3080
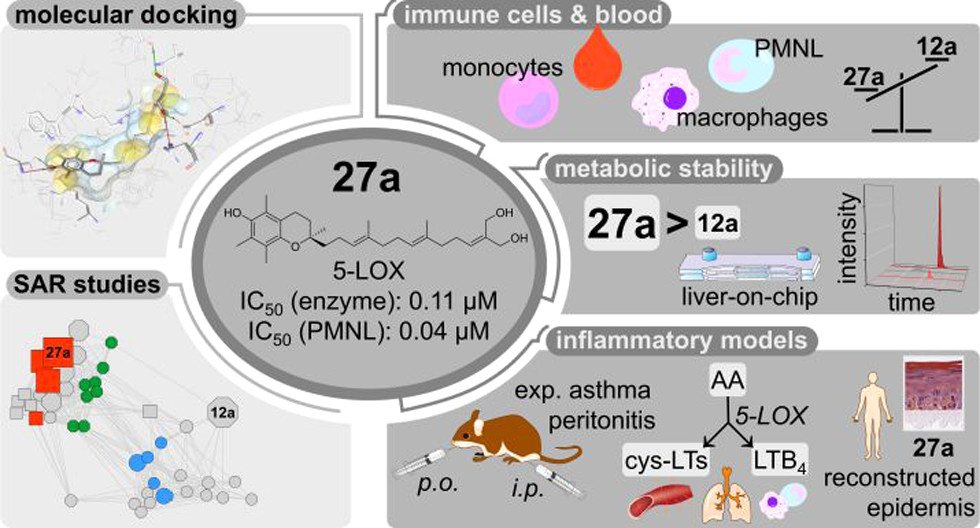
63. Exploration of long-chain vitamin E metabolites for the discovery of a highly potent, orally effective and metabolically stable 5-LOX inhibitor that limits inflammation
K. Neukirch, K. Alsabil, C.-P. Dinh, R. Bilancia, M Raasch, A. Ville, I. Cerqua, G. Viault, D. Bréard, S. Pace, V. Temml, E. Brunner, P. Jordan, M. Marques, K. Loeser, A. Gollowitzer, S. Permann, J. Gerstmeier, S. Lorkowski, H. Stuppner, U. Garscha, T. Rodrigues, G. Bernardes, D. Schuster, D. Seraphin, P. Richomme, A. Rossi, A. S. Mosig, F. Roviezzo, O. Werz, J.-J. Helesbeux, A Koeberle
J. Med. Chem. 2021, 64, 11496-11526
62. Facts and Figures on Materials Science and Nanotechnology Progress and Investment
S. Talebian*, T. Rodrigues*, J. das Neves, B. Sarmento, R. Langer, J. Conde
ACS Nano 2021, 15, 15940-15952
- *equal contribution
- selected for cover art
61. Augmenting adaptive machine learning with kinetic modelling for reaction optimization
A. F. Almeida, F. A. P. Ataíde*, R. M. S. Loureiro, R. Moreira, T. Rodrigues*
J. Org. Chem. 2021, 86, 14192-14198
- Invited contribution to the “Enabling Techniques for Organic Synthesis” issue
60. Combating small molecule aggregation with machine learning
K. Lee, A. Yang, Y.-C. Lin*, D. Reker, G. J. L. Bernardes, T. Rodrigues*
Cell Rep. Phys. Sci. 2021, 2, 100573
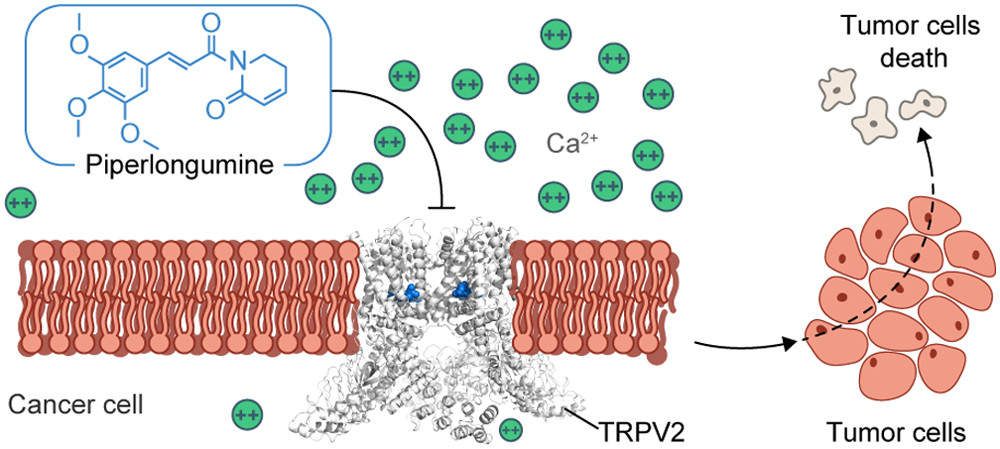
59. Allosteric Antagonist Modulation of TRPV2 by Piperlongumine Impairs Glioblastoma Progression
J. Conde*, R. A. Pumroy*, C. Baker*, T. Rodrigues*, A. Guerreiro, B. B. Sousa, M. C. Marques, B. P. de Almeida, S. Lee, E. P. Leites, D. Picard, A. Samanta, S. H. Vaz, F. Sieglitz, M. Langini, M. Remke, R. Roque, T. Weiss, M. Weller, Y. Liu, S. Han, F. Corzana, V. A. Morais, C. C. Faria, T. Carvalho, P. Filippakopoulos, B. Snijder, N. L. Barbosa-Morais, V. Y. Moiseenkova-Bell, Gonçalo J. L. Bernardes
ACS Cent. Sci. 2021, 7, 868-881
- *equal contribution
- highlighted in Público, GEN, ecancer, Technology networks, News Medical, Sciencemag, Physorg.com, Newswise, Mirage, Sciencenewsnet, EurekAlert! (21.04.2021)
- technology yielded TargTex S.A.
2020
58. Adaptive optimization of chemical reactions with minimal experimental information
D. Reker, Emily A. Hoyt, G. J. L. Bernardes*, T. Rodrigues*
Cell Rep. Phys. Sci. 2020, 1, 100247
- highlighted in Computational Chemistry Highlights blog (30.11.2018)
- highlighted in Proteins and Wave Functions (10.01.2019)
- highlighted in Chemistry World (12.11.2020)
- highlighted in editorial Cell Rep Phys Sci 2021, 2, 100316
- selected for “Best of 2020” collection
- most read in December 2020 to May 2021
57. Structural and biophysical insights of the mode of covalent binding of rationally designed potent BMX inhibitors
J. D. Seixas, B. B. Sousa, M. C. Marques, A. Guerreiro, R. Traquete, T. Rodrigues, I. S. Albuquerque, M. Sousa, A. R. Lemos, P. M. F. Sousa, T. M. Bandeiras, D. Wu, S. K. Doyle, C. V. Robinson, A. N. Koehler, F. Corzana, P. M. Matias, G. J. L. Bernardes
RSC Chem Biol 2020, 1, 251-262
- selected for Editor’s choice 2021
56. The good, the bad, and the ugly in chemical and biological data for machine learning
T. Rodrigues*
Drug Discov. Today: Technol. 2019, 32-33, 3-8
- highlighted in Practical Cheminformatics (18.01.2021)
55. The antidiabetic drug lobeglitazone has the potential to inhibit PTP1B activity
R. F. Rocha, T. Rodrigues, A. C. O. Menegatti, G. J. L. Bernardes, H. Terenzi
Bioorg. Chem. 2020, 100, 103927
54. Brain-sparing sympathofacilitators mitigate obesity without adverse cardiovascular effects
I. Mahú, A. Barateiro, E. Rial-Pensado, N. Martinéz-Sánchez, S. H. Vaz, P. M. S. D. Cal, B. Jenkins, T. Rodrigues, C. Cordeiro, M. F. Costa, R. Mendes, E. Seixas, M. M. A. Pereira, N. Kubasova, V. Gres, I. Morris, C. Temporão, M. Olivares, Y. Sanz, A. Koulman, F. Corzana, A. M. Sebastião, M. López, G. J. L. Bernardes, A. I. Domingos
Cell Metab. 2020, 31, 1120-1135
- highlighted in Nature Reviews Endocrinology (27.05.2020)
53. Machine learning for target discovery in drug development
T. Rodrigues*, G. J. L. Bernardes*
Curr. Opin. Chem. Biol. 2020, 56, 16-22
2019
52. Evaluation of linker length effects on a BET bromodomain probe
R. Traquete, E. Henderson, S. Picaud, P. M. S. D. Cal, F. Sieglitz, T. Rodrigues, R. Oliveira, P. Filippakopoulus, G. J. L. Bernardes
Chem. Commun. 2019, 55, 10128-10131
51. Synthetic organic chemistry driven by artificial intelligence
A. Filipa de Almeida, R. Moreira, T. Rodrigues*
Nat. Rev. Chem. 2019, 3, 589-604
- highlighted in Scale blog (27.08.2019)
50. Dissecting celastrol with machine learning to unveil dark pharmacology
T. Rodrigues*, B. P. de Almeida, N. L. Barbosa-Morais, G. J. L. Bernardes*
Chem. Commun. 2019, 55, 6369-6372
49. Natural product–drug conjugates for modulation of TRPV1-expressing tumors
C. Baker, T. Rodrigues, B. P. de Almeida, N. Barbosa-Morais, G. J. L. Bernardes
Bioorg. Med. Chem. 2019, 27, 2531-2536
48. Computational advances in combating colloidal aggregation in drug discovery
D. Reker*, G. J. L. Bernardes, T. Rodrigues*
Nat. Chem. 2019, 11, 402-418
2018
47. Discovery of 2,4-dimethoxypyridines as novel autophagy inhibitors
L. Robke, T. Rodrigues, P. Schröder, D. J. Foley, G. J. L. Bernardes, L. Laraia, H. Waldmann
Tetrahedron 2018, 74, 4531-4537
46. Machine intelligence decrypts β-lapachone as an allosteric 5-lipoxygenase inhibitor
T. Rodrigues*, M. Werner, J. Roth, E. H. G. da Cruz, M. C. Marques, S. A. Lobo, A. Koeberle, F. Corzana, E. N. da Silva Júnior, O. Werz, G. J. L. Bernardes*
Chem. Sci. 2018, 9, 6899-6903
- selected as hot article
- highlighted in Chemistry World (26.07.2018)
- interviewed on National TV December (Sic Notícias) 2018
- Exame Informática Award in “Software” category
45. Development of antibody-directed therapies: quo vadis?
T. Rodrigues*, G. J. L. Bernardes*
Angew. Chem. Int. Ed. 2018, 57, 2032-2034
2017
44. Chemoselective installation of amine bonds on proteins through aza-Michael ligation
A. Freedy, M. Matos, O. Boutureira, F. Corzana, A. Guerreiro, P. Akkapeddi, V. Somovilla, T. Rodrigues, K. Nicholls, B. Xie, G. Jiménez-Osés, K. Brindle, A. Neves, G. Bernardes
J. Am. Chem. Soc. 2017, 139, 18365-18375
43. Harnessing the potential of natural products in drug discovery from a cheminformatics vantage point
T. Rodrigues*
Org. Biomol. Chem. 2017, 15, 9275-9282
- selected as Hot article
- Invited contribution
42. A Water-Bridged Cysteine-Cysteine Redox Regulation Mechanism in Bacterial Protein Tyrosine Phosphatases
J. B. Bertoldo, T. Rodrigues, L. Dunsmore, F. A. Aprile, M. C. Marques, L. Rosado, O. Boutureira, T. B. Steinbrecher, W. Sherman, F. Corzana, H. Terenzi, G. J. L. Bernardes
Chem 2017, 3, 665-677
- highlighted at Medical Express (13.10.2017)
41. Vinyl ether/tetrazine pair for the traceless release of alcohols in cells
E. Jiménez-Moreno, Z. Guo, B. L. Oliveira, I. S. Albuquerque, A. Kitowski, A. Guerreiro, O. Boutureira, T. Rodrigues, G. Jiménez-Osés, G. J . L. Bernardes
Angew. Chem. Int. Ed. 2017, 56, 243-247
2016
40. Antibody-drug conjugates: The missing link
T. Rodrigues, G. J. L. Bernardes
Nat. Chem. 2016, 8, 1088-1090
39. Unveiling (-)-englerin A as a L-type calcium channel modulator
T. Rodrigues*, F. Sieglitz, V. J. Somovilla, P. M. S. D. Cal, A. Galione, F. Corzana*, G. J. L. Bernardes*
Angew. Chem. Int. Ed. 2016, 55, 11077-11081
38. From complex natural products to simple synthetic mimetics by computational de novo design
L. Friedrich, T. Rodrigues, P. Schneider, G. Schneider
Angew. Chem. Int. Ed. 2016, 55, 6789-6792
- Evaluated by the Faculty of 1000
37. Counting on natural products for drug design
T. Rodrigues, D. Reker, P. Schneider, G. Schneider
Nat. Chem. 2016, 8, 531-541
36. Natural product modulators of Transient Receptor Potential (TRP) channels as potential anti-cancer agents
T. Rodrigues, F. Sieglitz, G. J. B. Bernardes
Chem. Soc. Rev. 2016, 45, 6130-6137
35. Designing multi-target compound libraries with Gaussian process models
M. Bieler, M. Reutlinger, T. Rodrigues, P. Schneider, J. M. Kriegl, G. Schneider
Mol. Inf. 2016, 35, 192-198
2015
34. De novo fragment design for drug discovery and chemical biology
T. Rodrigues, D. Reker, M. Welin, M. Caldera, C. Brunner, G. Bagernet, P. Schneider, B. Walse, G. Schneider
Angew. Chem. Int. Ed. 2015, 54, 15079-15083
- selected as Very Important Paper
- highlighted in “In The Pipeline” (2.11.2015)
- highlighted in “Practical Fragments” (23.11.2015)
33. Revealing the macromolecular targets of fragment-like natural products
T. Rodrigues, D. Reker, J. Kunze, P. Schneider, G. Schneider
Angew. Chem. Int. Ed. 2015, 54, 10516-10520
32. Fragment-based de novo design reveals a small molecule inhibitor of Helicobacter pylori HtrA
A. M. Perna*, T. Rodrigues*, T. P. Schmidt, M. Böhm, K. Stutz, D. Reker, B. Pfeiffer, K.-H. Altmann, S. Backert, S. Wessler, G. Schneider
Angew. Chem. Int. Ed. 2015, 54, 10244-10248
- *equal contribution
- selected as Hot Paper
31. Repurposing de novo designed entities reveals phosphodiesterase 3B and cathepsin L modulators
T. Rodrigues, Y.-C. Lin, M. Hartenfeller, S. Renner, Y. F. Lim, G. Schneider
Chem. Commun. 2015, 51, 7478-7481
30. Multidimensional de novo design reveals 5-HT2B receptor-selective ligands
T. Rodrigues, N. Hauser, D. Reker, M. Reutlinger, T. Wunderlin, J. Hamon, G. Koch, G. Schneider
Angew. Chem. Int. Ed. 2015, 54, 1551-1555
Prior to 2015
29. Revealing the macromolecular targets of complex natural products
D. Reker, A. M. Perna, T. Rodrigues, P. Schneider, M. Reutlinger, B. Mönch, A. Koeberle, C. Lamers, M. Gabler, H. Steinmetz, R. Müller, M. Schubert-Zsilavecz, O. Werz, G. Schneider
Nat. Chem. 2014, 6, 1072-1078
- highlighted in ETH Life, Science Daily, Physorg.com, EurekAlert! (12.11.2014)
- highlighted in the Chimia issue of March 2015
- evaluated by the Faculty of 1000
28. Coping with polypharmacology by computational medicinal chemistry
G. Schneider, D. Reker, T. Rodrigues, P. Schneider
Chimia 2014, 68, 648-653
- Invited contribution to special issue Computational Chemistry in Switzerland
27. Antiplasmodial drugs in the gas phase: A CID and DFT study of quinolon-4(1H)-imine derivatives
P. J. A. Madeira, A. R. F. Sitoe, D. Gonçalves, T. Rodrigues, R. C. Guedes, F. Lopes, R. Moreira, M. R. Bronze
J. Am. Soc. Mass Spectrom. 2014, 25, 1650-1661
26. Accessing new chemical entities using microfluidic technology
T. Rodrigues, P. Schneider, G. Schneider
Angew. Chem. Int. Ed. 2014, 53, 5750-5758
25. Identifying the macromolecular targets of de novo designed chemical entities through self-organizing map consensus
D. Reker, T. Rodrigues, P. Schneider, G. Schneider
Proc. Natl. Acad. Sci. USA 2014, 111, 4067-4072
- highlighted in ETH Life (17.03.2014)
- highlighted in ScienceWorld Report, Science Daily, physorg.com, Ad hoc news, EurekAlert! (18.03.2014)
24. Targeting dynamic pockets of HIV-1 protease by structure-based computational screening for allosteric inhibitors
J. Kunze, N. Todoroff, P. Schneider, T. Rodrigues, T. Geppert, F. Reisen, H. Schreuder, J. Saas, G. Hessler, K.-H. Baringhaus, G. Schneider
J. Chem. Inf. Model. 2014, 54, 987-991
23. Multi-objective molecular de novo design by fragment prioritization
M. Reutlinger, T. Rodrigues, P. Schneider, G. Schneider
Angew. Chem. Int. Ed. 2014, 53, 4244-4248
- highlighted in ETH Life 17.03.2014
22. Combinatorial chemistry by ant colony optimization
J. A. Hiss, M. Reutlinger, C. P. Koch, A. M. Perna, P. Schneider, T. Rodrigues, S. Haller, G. Folkers, L. Weber, R. B. Baleeiro, P. Walden, P. Wrede, G. Schneider
Future Med. Chem. 2014, 6, 267-280
21. Combining on-chip synthesis of a focused combinatorial library with computational target prediction reveals imidazopyridine GPCR ligands
M. Reutlinger, T. Rodrigues, P. Schneider, G. Schneider
Angew. Chem. Int. Ed. 2014, 53, 582-585
20. Flashback forward: Reaction-driven de novo design of bioactive compounds
T. Rodrigues, G. Schneider
Synlett 2014, 25, 170-178
19. Flavones as isosteres of 4(1H)-quinolones: discovery of ligand efficient and dual stage antimalarial lead compounds
T. Rodrigues*, A. S. Ressurreição, F. P. da Cruz, I. S. Albuquerque, J. Gut, M. P. Carrasco, D. Gonçalves, R. C. Guedes, D. J. V. A. dos Santos, M. M. Mota, P. J. Rosenthal, R. Moreira, M. Prudêncio, F. Lopes
Eur. J. Med. Chem. 2013, 69, 872-880
18. Steering target selectivity and potency by fragment-based de novo drug design
T. Rodrigues, T. Kudoh, F. Roudnicky, Y. F. Lim, Y.-C. Lin, C. P. Koch, M. Seno, M. Detmar, G. Schneider
Angew. Chem. Int. Ed. 2013, 52, 10006-10009
- selected as Very Important Paper
- highlighted in the Chimia issue of December 2013
17. Exploring the Molecular Basis of Qo bc1 Complex Inhibitors Activity to Find Novel Antimalarials Hits
M. P. Carrasco, J. Gut, T. Rodrigues, F. Lopes, P. J. Rosenthal, R. Moreira, D. J. V. A. dos Santos
Mol. Inf. 2013, 32, 659-670
16. Quinolin-4(1H)-imines are Potent Antiplasmodial Drugs Targeting the Liver Stage of Malaria
T. Rodrigues*, F. P. da Cruz, Maria J. Lafuente-Monasterio, D. Gonçalves, A. S. Ressurreição, A. R. Sitoe, M. R. Bronze, J. Gut, G. Schneider, M. M. Mota, P. J. Rosenthal, M. Prudêncio, F.-J. Gamo, F. Lopes, R. Moreira
J. Med. Chem. 2013, 56, 4811-4815
15. Chemically advanced template search (CATS) for scaffold-hopping and prospective target prediction for ‘orphan’ molecules
M. Reutlinger, C. P. Koch, D. Reker, N. Todoroff, P. Schneider, T. Rodrigues, G. Schneider
Mol. Inf. 2013, 32, 133-138
14. De novo design and optimization of Aurora A kinase inhibitors
T. Rodrigues, F. Roudnicky, K. P. Koch, T. Kudoh, D. Reker, M. Detmar, G. Schneider
Chem. Sci. 2013, 4, 1229-1233
13. Drugs by numbers: Reaction-driven de novo design of potent and selective anticancer leads
B. Spänkuch, S. Keppner, L. Lange, T. Rodrigues, H. Zettl, C. P. Koch, M. Reutlinger, M. Hartenfeller, P. Schneider, G. Schneider
Angew. Chem. Int. Ed. 2013, 52, 4676-4681
- selected as Very Important Paper
12. Significance estimation for sequence-based chemical similarity searching (PhAST) and application to Aurora A kinase inhibitors
V. Hähnke, N. Todoroff, T. Rodrigues, G. Schneider
Future Med. Chem. 2012, 4, 1897-1906
11. Drug screen targeted at Plasmodium liver stages identifies a potent multi-stage anti-malarial drug
F. P. da Cruz, C. Martin, K. Buchholz, M. J. Lafuente-Monasterio, T. Rodrigues, B. Sönnichsen, R. Moreira, F.-J. Gamo, M. Marti, M. M. Mota, M. Hannus, M. Prudêncio
J. Infect. Dis. 2012, 205, 1278-1286
- Evaluated by the Faculty of 1000
10. Targeting the Liver Stage of Malaria Parasites: A Yet Unmet Goal
T. Rodrigues, M. Prudêncio, R. Moreira, M. M. Mota, F. Lopes
J. Med. Chem. 2012, 55, 995-1012
9. From virtual screening to bioactive compounds by visualizing and clustering of chemical space
A. Klenner, V. Hähnke, T. Geppert, P. Schneider, H. Zettl, S. Haller, T. Rodrigues, F. Reisen, B. Hoy, A. M. Schaible, O. Werz, S. Wessler, G. Schneider
Mol. Inf. 2012, 31, 21-26
8. Microwave-assisted Wittig reaction of semistabilized nitro-substituted benzyltriphenyl-phosphorous ylides with aldehydes in phase-transfer conditions
T. Rodrigues, F. Lopes, R. Moreira
Synth. Commun. 2012, 42, 747-755
7. Identification of new antimalarial leads by use of virtual screening against cytochrome bc1
T. Rodrigues, J. Gut, P. J. Rosenthal, P. M. O’Neill, G. A. Biagini, R. Moreira, F. Lopes, D. J. V. A. dos Santos, R. C. Guedes
Bioorg. Med. Chem. 2011, 19, 6302-6308
6. New hope in the fight against malaria?
T. Rodrigues*, R. Moreira, F. Lopes
Future Med. Chem. 2011, 3, 1-3
5. A quantum mechanical study of novel potential inhibitors of cytochrome bc1 as antimalarial compounds
T. Rodrigues, D. J. V. A. dos Santos, R. Moreira, F. Lopes, R. C. Guedes
Int. J. Quantum Chem. 2011, 111, 1196-1207
4. Inhibitors of the Mitochondrial Electron Transport Chain and de novo Pyrimidine Biosynthesis as Antimalarials: The Present Status
T. Rodrigues, F. Lopes, R. Moreira
Curr. Med. Chem. 2010, 17, 929-956
3. Design, synthesis and Structure Activity-Relationships of (1H-pyridin-4-ylidene)amines as potential antimalarials
T. Rodrigues, R. C. Guedes, D. J. V. A. dos Santos, M. Carrasco, J. Gut, P. J. Rosenthal, R. Moreira, F. Lopes
Bioorg. Med. Chem. Lett. 2009, 19, 3476-3480
2. Bis{(E)-3-[(diethylmethylammonio)methyl]-N-[3-(N,N-dimethylsulfamoyl)-1-methylpyridin-4-ylidene]-4-methoxyanilinium} tetraiodide pentahydrate
T. Rodrigues, R. Moreira, B. Dacunha-Marinho, F. Lopes
Acta Cryst. E 2009, E65, o283-o284
1. Unanticipated Acyloxymethylation of Sumatriptan Indole Nitrogen Atom and its Implications in Prodrug Design
T. Rodrigues, R. Moreira, R. C. Guedes, J. Iley, F. Lopes
Arch. Pharm. Chem. Life Sci. 2008, 341, 344-350